Nitrogen Content of Soils and Factors Affecting Soil Nitrogen
Soil nitrogen (N) is the most abundant element in the atmosphere and is usually the most limiting crop nutrient. Nitrogen cycles through soil in various processes and forms. Some processes are necessary to convert N into forms which plants can use.
Some processes can lead to N losses such as leaching or volatilization. Nitrogen is added to soil naturally from N fixation by soil bacteria and legumes and through atmospheric deposition in rainfall.
Additional N is typically supplied to the crop by fertilizers, manure, or other organic materials. Soil nitrate-N is an excellent indicator of N-cycling in soils, whether carryover nitrogen was used by the previous crop and whether additional nitrogen is needed.
Nitrogen Content of Soils
Improved agricultural methods and better crop varieties are demanding more and more of this element. Soils alone seldom can meet the increased demand because they were never well supplied with nitrogen or because they have lost much of their original supply during 50 to 100 years of cultivation.
Nitrogen is of special importance because plants need it in rather large amounts, it is fairly expensive to supply, and it is easily lost from the soil. A major factor in successful farming is the farmer’s ability to manage nitrogen efficiently.
Losses of Nitrogen from Soil-Plant System
Apart from nitrate (NO3 – N) and ammonium (NH4+ -N) which are plant utilizable forms of nitrogen in the soil, other forms of inorganic N such as nitrogen dioxide (NO2), nitric oxide (NO) ammonia (NH3), nitrous oxide N 20 and nitrogen gas (N2) do exist having been transformed from organic fractions.
However, these other forms usually escape to the atmosphere in gaseous forms completely away from the soil-plant system. They are regarded as nitrogen losses and are usually the products of denitrification and volatilisation.
Other losses are through crop removal or by harvest, erosional losses and liquid leaching losses.Certain portions of nitrogen (NH4 – N and simple amino compounds) are held on negatively charged sites in the soil in the interlayer spaces of 2:1 clay minerals and are also unavailable to crops but cannot be regarded as losses since they can still be released for plant use under certain soil conditions.
Inherent Factors Affecting Soil Nitrogen
Inherent factors such as soil drainage, soil texture, and slope steepness impact N-transport and N transformation processes that limit availability to crops or lead to losses.
Inherent factors such as rainfall and temperature; and site conditions such as moisture, soil aeration (oxygen levels), and salt content (electrical conductivity/EC) affect rate of N mineralization from organic matter decomposition, nitrogen cycling, and nitrogen losses through leaching, runoff, or denitrification.
Organic matter decomposes releasing N more quickly in warm humid climates and slower in cool dry climates. This N release is also quicker in well aerated soils and much slower on wet saturated soils.
Read Also : Sulphur Content of Soils, Forms and Functions of Sulphur in Plants
How Leaching affects Soil Nitrogen
Nitrogen can readily leach out of the root zone in nitrate-N form. The potential for leaching is dependent on soil texture (percentage of sand, silt, and clay) and soil water content.
Water moves more quickly through large pore spaces in a sandy soil than it does through small pores in a clayey soil and water holding capacity is much lower in sandy soils, making them especially vulnerable.
Soils that have poor drainage and are ponded or saturated with water causes denitrification to occur resulting in loss of N as a gas which can result in emission of potent greenhouse gases, yield reduction and increased N fertilizer expense.
Soil Nitrogen Management
Management factors, such as N-rate, N source, N placement method, timing of application, irrigation management, residue management, crop type, etc. all can affect how efficiently N is used by crops and amount of N losses.
Nitrogen management on sandy soils is important because of high potential for leaching losses. Selecting appropriate N rate is the primary management consideration.
However, nitrogen source, timing N application close to plant uptake, and method of application such as injecting N to avoid losses are also important.
Management measures that increase organic matter and avoid compaction are also important to stabilize crop N supply, increase aeration, and to limit N losses due to denitrification occurring in saturated soils.
Nitrogen Supply to Soils
Nitrogen rates should be based on amount needed to optimize yield based on agronomic economic and environmental considerations.
When planning N-fertilizer or manure application rates appropriate N-credits should be accounted for including; soil test residual nitrate-N, soil organic matter mineralization, legume credits, manure or other organic amendments, irrigation water nitrate-N, residue decomposition and natural N sources.
Time N fertilization to provide adequate amounts of N when plants are actively growing and using N rapidly. Losses of applied N from fertilizer can be reduced by delaying application until the crop has emerged (side dressing).

Split N applications, where some N is applied prior to crop emergence and the balance after emergence can increase crop N-use efficiency.
Fertilizer source is important to increase N recovery by crops, avoid N-loss from volatisation and be matched to the type of placement method to reduce losses and maximize recovery by crops.
Anhydrous ammonia is usually the least expensive N source, but this material must be handled safely, and must be injected/knifed in with ideal soil moisture conditions. Urea and urea containing materials should be injected to reduce loss from ammonia volatilization.
Surface applied urea N fertilizers, should not be applied during warm humid conditions, or on wet residues because of high potential for N losses from volatisation. Manure or organic amendments can be an effective N-fertilizer.
However, care must be taken to apply manure uniformly at a known rate, and account for mineralization rate. Placement of N-fertilizer can be accomplished by several methods.
Methods of Application
Typical methods include:
Side dress applications after crop emergence,
Knifed application placing a band of fertilizer below the soil surface,
Broadcast applications that uniformly distribute N, and
Through sprinkler irrigation systems.
Each placement method has its advantages, and must be matched to type of fertilizer or manure that will be applied. Irrigation scheduling is important.
The goal is to supply enough water to optimize yield while avoiding excess irrigation which can increase costs and leach N below the root zone.
Keys to Managing N in Most Efficient Manner include these Strategies:
Apply recommended rate based on realistic yield.
Time N application just before peak crop demand.
Select an ammonium containing fertilizer which provides greater N recovery by crops.
Inject N if possible to avoid ammonia or volatisation losses.
Use N-inhibitors when N is applied outside of growing season.
Credit all sources of N.
Irrigate wisely.
Monitor crop nitrogen needs by scouting.
Regular soil testing for nitrate (including deep samples), and soil salt content (EC).
Symptom of Nitrogen Deficiency in Crops
Yellow coloration in a “V” shaped pattern is symptomatic of nitrogen deficiency. This pattern progresses from leaf end to leaf collar and from lower to upper leaves. Lower leaves often die when nitrogen deficiency is severe.
Biological Nitrogen Fixation
Biological nitrogen fixation is the conversion of dinitrogen (N2) in soil air into combined forms useable by the plants and effected by microorganisms.
Some groups of microbes live freely in the soil where they are able to convert N2 into body tissue nitrogen form and when they die and decompose, the combined nitrogen is released for plant use.
These groups of free living N-fixers are known as Non-symbiotic nitrogen fixing microorganisms. In the tropics Azotobacterand Bjeirinckiaspecies are known to be free-living microbes that fix N2 under aerobic well aerated soils of pH 6.0.
Clostridium spp. are free living bacteria that fix N2 under anaerobic conditions in soil. Other living soil micro-organisms that fix nitrogen are blue -green algae and Azospiriullumin the rhizosphere of certain plants. The range of fixed N by these group of non-symbiotic microbes is between 2-25 kg/ha/year.
In symbiotic fixation, bacterial and actinomycetes effect the formation of root nodules (abnormal root growth) in both legume and non-legume plants and then inhabit those nodules where they fix nitrogen.
The host plant supplies the bacteria with carbohydrate as source of energy, and the bacteria supplies the plant with fixed nitrogen compounds.in effect, both the plant and the microorganism have a mutually beneficial association known as symbiosis.
There are various species of Rhizobium bacteria that inhabit different plant species such as R.trifolicwhich inhabit clovers, R.japonicumfor Glycinemax(soyabeans) and R.Phaseoliwhich associates with Phaseolusvulgaris(dry beans).
Appropriate Rhizobia cultures are used to inoculate the soil for a particular crop to nodulate for the fixation of nitrogen.
The Nitrogen Cycle
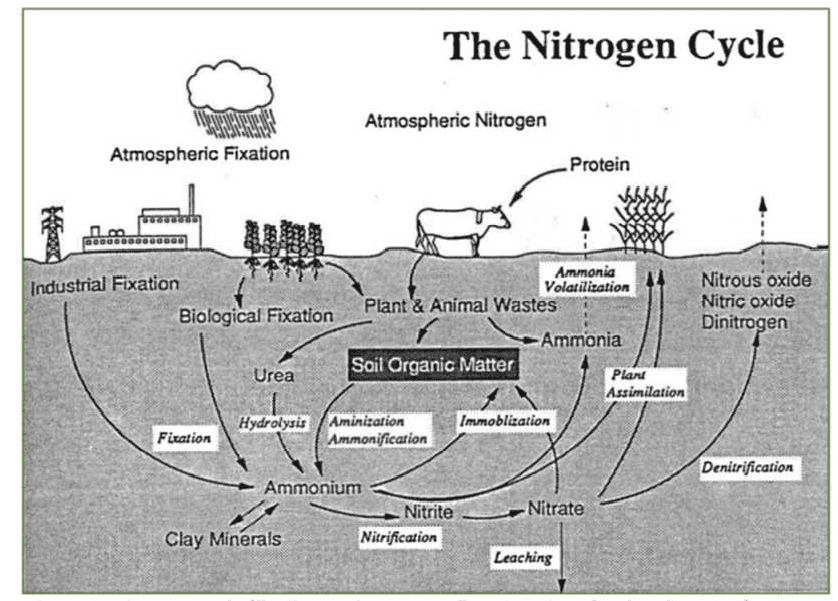
Besides nitrogen (N2) gas within soil pore space, nitrogen is found in both organic and inorganic forms in soil. Organic forms occur in soil organic matter which consists of three primary parts including small (fresh) plant residues and small living soil organisms, decomposing (active) organic matter, and stable organic matter.
Predominate inorganic forms of N in soils are ammonium (NH4) and nitrate (NO3), which are both useable by plants. The nitrogen cycle illustrates reactions that various inorganic and organic N compounds undergo in soil.
The nitrogen cycle typically begins with nitrogen in its simplest stable form, dinitrogen (N2) in air, and follows it through the processes of fixation, mineralization, nitrification, leaching, plant assimilation, ammonia volatilisation, denitrification, and immobilization.
In summary, Nitrogen (N) is the most abundant element in the atmosphere and is usually the most limiting crop nutrient. Nitrogen cycles through soil in various processes and forms.
Some processes can lead to N losses such as leaching or volatilization. Nitrogen is added to soil naturally from N fixation by soil bacteria and legumes and through atmospheric deposition in rainfall.
An abundant supply of the essential nitrogen compounds is required in each plant cell for a good rate of reproduction, growth, and respiration. Even the green leaf pigment chlorophyll, which enables plants to use the energy of sunlight to form sugars, starches, and fats from carbon dioxide and water, is a nitrogenous compound.
Read Also : The Concept of Animal Production on Agriculture









