Phosphorus Content of Soils and Factors Influencing Phosphorus Availability in the Soil
Phosphorus constitutes about 0.2 percent of a plant’s dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP).
After nitrogen (N), phosphorus (P) is the second most limiting nutrient. It can reduce plant growth and development and potentially limit crop yield. However, excess phosphorus in soil can be detrimental to the environment because it can enter freshwater bodies through surface runoff and can cause algal bloom reducing water quality.
Improved phosphorus management can create profitable crop production systems while reducing negative impacts on the environment. The objective of this document is to understand phosphorus forms, transformation, and cycling in the soil.
Phosphorus cycle is unique and different from the nitrogen cycle because phosphorus does not exist in a gaseous form. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production.
Phosphorous Forms Present in the Soil
Soil phosphorus is found in two forms, namely organic and inorganic. These two forms together make up the total soil phosphorus.
Although total soil phosphorus is generally high, with concentrations ranging from 200 to 6,000 pounds per acre, 80 percent of this phosphorus is immobile and not available for uptake by the plant.
Approximately 30 to 65 percent of total soil phosphorus is in organic forms, which are not plant available, while the remaining 35 to 70 percent is in inorganic forms.
Organic forms of phosphorus include dead plant/animal residues and soil micro- organisms. Soil micro-organisms play a key role in processing and transforming these organic forms of phosphorus into plant available forms. The inorganic phosphorus forms can be classified to exist in three different pools:
Plant-available (soil solution) phosphorus: This pool is comprised of inorganic phosphorus dissolved in water/soil solution that is readily available for plant uptake.
Sorbed phosphorus: This phosphorus pool is comprised of inorganic phosphorus attached to clay surfaces, iron (Fe), aluminum (Al), and calcium (Ca) oxides in soil. The phosphorus in this pool is released slowly for plant uptake.
Mineral phosphorus: This phosphorus pool is comprised of primary and secondary phosphate minerals present in soil.
Examples of primary phosphorus minerals include apatite, strengite, and variscite. The secondary phosphorus minerals include calcium (Ca), iron (Fe), and aluminum (Al) phosphates. The release of phosphorus from this pool is extremely slow and occurs when the mineral weathers and dissolves in soil water.
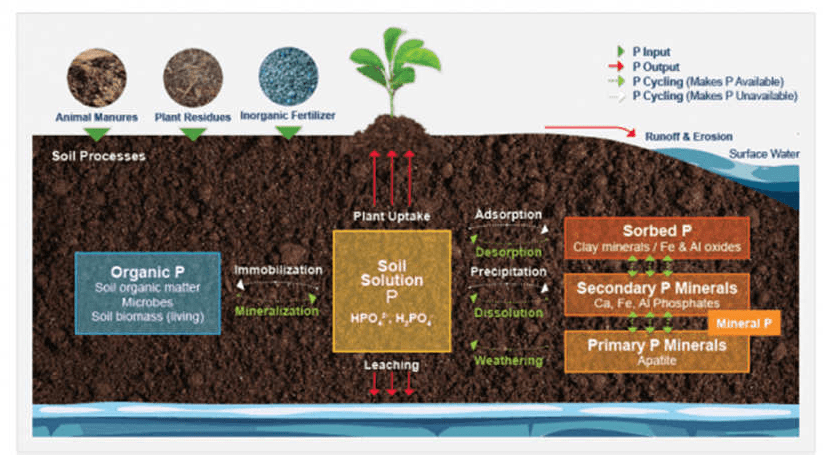
Phosphorus Cycling and Transformation in the Soil
Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Following are explanations of these processes:
Mineralization and Immobilization
Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. Immobilization, on the other hand, is the reverse of mineralization.
During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. Immobilization typically occurs when crop residues are incorporated in the soil.
As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization.
Since mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature, pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc.
Read Also : Potassium Content of Soils, Forms, Sources, Relationships and Deficiency Symptoms
Adsorption and Desorption
Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. The phosphorus binding takes place on clay surfaces or the iron (Fe) and aluminum (Al) oxides and hydroxides present in soil.
Adsorption is a fast process and reversible in nature, meaning that adsorbed phosphorus can be released into soil solution via a process known as desorption and will be available for plant uptake.
Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. Another soil property that favors phosphorus adsorption is the clay content.
Soils with greater clay content have higher adsorption capacity than coarse textured sandy soils.
Weathering, Precipitation, and Dissolution
Soil contains minerals that are rich in phosphorus. These minerals are classified into primary and secondary minerals. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake.
Primary minerals such as apatite are very stable and resistant to weathering. Hence, phosphorus is released very slowly compared to secondary phosphorus minerals such as calcium, iron, or aluminum phosphates.
Precipitation on the other hand is a process by which metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates.
Precipitation is a slow process and involves a permanent change into metal phosphates. These metal phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very slow.
Dissolution is a form of weathering when the phosphate minerals dissolve and release phosphate back into the soil solution.
Phosphorus Loss
Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching. Surface runoff is the major pathway for phosphorus loss from soils.
Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. In general, phosphorus loss by leaching is minimal compared to surface runoff.
Factors Influencing Phosphorus Availability in the Soil
While the processes such as weathering, dissolution, mineralisation, and desorption increase phosphorus availability in the soil for plant uptake, processes such as immobilisation, adsorption, precipitation, runoff, and erosion decrease the phosphorus availability.
In addition, phosphorus availability in soil solution is influenced by the following factors:
Organic Matter: Organic matter is an important factor in controlling phosphorus availability. With the addition of organic matter, availability of phosphorus increases. This is due to the following reasons:
Mineralization of organic matter releases plant- available forms of phosphorus into soils.
Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. This process will increase availability of phosphorus.
Clay Content: Soils with higher clay content have high phosphorus retention capacity because clay particles have very large surface area per unit volume, which can adsorb phosphorus easily.
Soil Mineralogy: The mineral composition of the soil influences the phosphorus adsorption capacity. For example, soils with a high content of Al3+ and Fe3+also tend to have the greatest phosphorus adsorption capacity.
SoilpH: Optimum soil pH between 6 and 7 will result in maximum phosphorus availability. At low pH (acidic soils), soils have greater amounts of aluminum and iron, which form very strong bonds with phosphate. At high pH when calcium is the dominant cation, phosphate tends to precipitate with calcium.
Other factors:Temperature, moisture, and soil aeration can affect the rate of P mineralisation from organic matter decomposition. For example, in warm, humid climates organic matter decomposes faster compared to cool dry climates.
Phosphorus Deficiency and Toxicity
Since P is a mobile element, deficiency symptoms first show up in older leaves of plants. The deficiency symptoms may appear as:
Stunted overall growth of whole plant compared to normal plants.
Dark green colour, dark red to purple discoloration of stems, and, at times, dull green.
Protein synthesis is impaired, vegetative growth is depressed.
P-deficient plants have limited root system and thin stems. In cereals, tillering is affected.
There is a deposition of starch in roots.
Stems of annual plants have reddish green colour because of formation of anthocyamins.
Leaves are tinged with brownish colour and fall off prematurely.
Phosphorus contents of P-deficient plants are usually low (0.1% P).
When cereal and herbage are supplied with P, their P-content may go up to about 0.3 to 0.4%. Phosphorus toxicity is not common, but when it occurs it leads to reduced growth due to retardation of up-take and translocation of micro-nutrients including zinc, iron and copper.
P-Management Strategies
Four major P-management strategies are:
Lime acid soils to increase soil pH to between 6.5 and 7.0;
Apply small amounts of P fertilizer frequently rather than large amounts at one time;
Reduce P tie-up by banding/injecting P fertilizer or liquid manure; and
Place P fertilizers near crop row or in furrow where roots are most active.
In summary, various components of phosphorus cycle in soil can be correlated with the types of money in your bank. Just as money can be separated into categories: savings or checking accounts, the checks you carry for use as needed, and the cash you keep with you; phosphorus in soil can also be categorized to exist in three different accounts/pools.
Soil phosphorus is found in two forms, namely organic and inorganic. These two forms together make up the total soil phosphorus. The inorganic P can be classified to exist in the following forms; Plant-available (soil solution) phosphorus, Sorbed phosphorus, and Mineral phosphorus.
Factors influencing phosphorus availability in the soil include; organic matter, clay content, soil mineralogy, soil pH and other factors.
Read Also : Comprehensive Farming Guide For Starters









