Fish age and growth are critical correlates with which to evaluate many other biological (and pathological) processes, such as productivity, yield per recruit, prey availability, habitat suitability and even feeding kinematics.
While age and growth are always used together in phraseology, it is important to remember that each term has its own distinct meaning, which was eloquently stated by DeVries and Frie (1996):
“Age refers to some quantitative description of the length of time that an organism has lived, whereas growth is the change in body or body part size between two points in time, and growth rate is a measure of change in some metric of fish size as a function of time.”
Methods of Fish Age and Growth Studies
Steps Involved in Fish Age and Growth Studies
Collect a random sample of fish and this should include representative of all sizes and age of fish species present in the population.
For each species, record the date of collection, habitat and method of collection.
Measure the length and weight of each species, dissect, and determine the sex and stages of gonad development.
Collection from each the structures that will be used for age determination.
Determine the age of each specimen using more than one method of age.
Calculate growth for each sex separately and for combined sexes if the calculation for individual method is significant different using chi-square. If the different are significant both sexes can be combined.
Determine length-weight relationship for each sex separately and for combined sexes.
Calculate the condition factor according to age, size, sex and season.
Precise and accurate age information is the key to obtaining quality estimates of growth and other vital rates such as natural mortality and longevity, and is essential for successful fisheries management. The effect of inaccurate age determinations on population dynamics studies can lead to serious errors in stock assessment resulting in overexploitation.
The determination of fish age and growth is fundamental in fisheries biology and management. Such age-determined parameters as mortality and growth underlie the population dynamics models used in fishery analyses.
Age studies can furnish other basic data such as stock age structure, age at first maturity, spawning frequency, individual and stock responses to changes in the habitat, recruitment success, etc. Age and growth data also permit the determination of population changes due to fishing rates.
Although numerous methods have been used to age fish, three general methods predominate:
Anatomical method: It is possible to determine the age of fish by evaluation of growth rings forming bony structures such as scales, otoliths, the opercular bone, the vertebrae and the cross section of dorsal or pectoral fin rays.
Ages of fish are estimated by comparison of the readings from various bony structures and different readers Furthermore, ageing errors must be considered before deciding on the most reliable bony structure for ageing fish.
Two important considerations when selecting a structure for aging a sample of fish are whether the structure yields accurate estimates of fish age and whether the structure can be obtained without killing the specimens.
Other researchers have used radiochemistry as an alternative to physically age fish by measuring radioactive decay of elements present in the bony structures, with often quite conflicting results.
Length-frequency analysis: It determines age by plotting length-frequency distributions (also known as the Petersen Method) from the population to reveal peaks assumed to represent average lengths for each separate age class.
If the populations of fish that are characterized by regular influences of new recruits are adequately sampled, they usually reveal a size structure featuring a train of length-frequency polygons especially in the first year(s) of life, which indicates the presence of several age groups.
This method requires individual lengths of large number of fish population and little overlap in the sizes of fish in adjacent age groups and is best used for fish that are fast growing with short spawning periods and short life spans.
Using length-frequency data to age fish is relatively simple and inexpensive, however, the life history traits (fast growth and short spawning seasons) needed for accurate analysis are not typical of many species, for example, tropical reef fishes such as snappers and groupers.
This method involves monitoring the progression through time of the identifiable modes in size classes. It cannot be used to determine the age of an individual fish.
Direct estimate: This is achieved through direct measurements of growth rate of specific specimens extrapolated to the stock as a whole.
Marking and subsequent recapture of fish, or monitoring the growth of captive fish of known age are two direct estimation methods. Mark/recapture approaches have been used to estimate growth, movement, and population size for many fishes.
Fish are marked externally by fin clipping or through the attachment of a visible tag. Internal tags can be used as well.
Assumptions are made with any tagging study that tags will not cause changes in growth, feeding, reproduction, movement, or survival of the fish and that all tags will remain attached to the fish. Directly observing growth of laboratory reared fishes can also be used to assign growth rates to fish of known ages.
Although accurate, these two methods of observing growth can become expensive, time consuming, and labor intensive, especially with fish that are extremely sensitive to changes in habitat. They are the most accurate method of age determination under natural condition.
Reliable age estimates, however, are difficult to obtain for species found in tropical regions; tropical systems are characterized by species with fast growth, high productivity and high turn- over, that is, short lives.
In seasonal tropical climate, the patterns of marks on hard part of fish may be difficult to interpret and unreliable for age determination. Besides, many tropical fishes are known to have annual peaks in spawning activities which are reflected in other length- frequency distribution.
Definition and Designation of Fish Age
‘Age-group’ refers to age in years, while ‘year-class’ refers to the fish produced in a given year. There is yet to be a complete agreement on age designation or terminologies.
The age of a fish is usually designated by reference to the annual marks on its hard structure such as otoliths and to some extent the season of the year, rather according to its exact length of life (which is not precisely known).
A fish in its first growing season belong to the age-group 0, and its successive stages may be called a larval (fry or sac-fry) and fingerlings (young -of -the –year or under yearling).
Read Also : Guide to Proper Artificial Insemination in Farm Animals
A fish in its second growing season is said to be a member of the age-group 1 and maybe called a yearling. In the third growing season the fish is age 2 and is called a 2-year-old and so on.
A yearling will typically have one annual mark on its hard structure. However, in some species, the time when the annual marks appear may vary for as much as two or three months.
The Use of Scales to Age Fish
Scales of fishes are remarkable structures. Much information can be obtained about the growth history and longevity of individual fish by close examination of their scales or other bony structures. On the population level, also, age and growth is an excellent index to well-being.
Scales are bony structures that grow shingle-like from pockets within the skin. Scales are covered with a very thin, outer layer of skin called the epidermis.
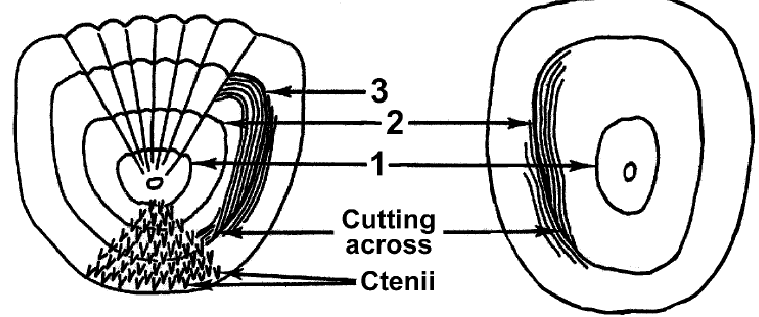
Among fishes there are basically two kinds of scales: the ctenoid scale found on spiny-rayed fishes such as bass, sunfish, perch, and the cycloid scale found on soft-rayed fishes such as trout, suckers.
The ctenoid scale has small, sharp projections (ctenii) which give a rough texture to spiny-rayed fish. The cycloid scale lacks ctenii; thus soft-rayed fish tend to be smooth textured.
Scales start to form when a fish is about an inch long. The number of scales covering the body remains constant throughout life, and in general, scale growth is proportional to fish growth. As the scale grows, circuli (ridges) form on the edge.
Circuli form a concentric pattern over the course of a year that is related to environmental and growth conditions. During the colder months, when fish eat little and growth ceases, the circuli are crowded together and may be incomplete.
Annuli (true year marks) are characterized by crowded ridges and consistent “cutting across” at both sides of the scale
Unusual events may cause false annuli to form on scales. Examples are extreme water temperatures, injury, or any other stress that causes growth to stop for a period of time during the normal growing season.
False annuli may be very similar in appearance to true annuli, but often “cut across” on only one side of the scale or are not evident on all scales from a particular fish.
Old fish are often under-aged from scales. As a fish becomes older, growth rate slows down and annuli become closer together. The result is that it is difficult (sometimes impossible) to recognize the most recent annuli on very old fish scales.
Some fish (such catfish) have no scales, and other species (such as bowfin) have non-recognizable pattern on their scales. For those fish, a cross-section of a spine or a vertebra should be examined for age rings similar to rings on trees.
Ear bones (otoliths), spines, and vertebrae are also more reliable than scales for aging, perch, bass, sucker, pikes, and salmon.
Procedures
Recording data on scale envelopes
Record accurate and complete information on the scale envelope. Give the following information:
Species – Give common name of fish.
Locality – Give the name of the lake or stream from which fish was taken.
County – The name of the county in which lake or stream is located.
T.R.Sec – Give the Township, Range, and Section in which body of water is located. This is especially needed when two lakes with the same name occur in the same county.
Date – Date when fish was collected.
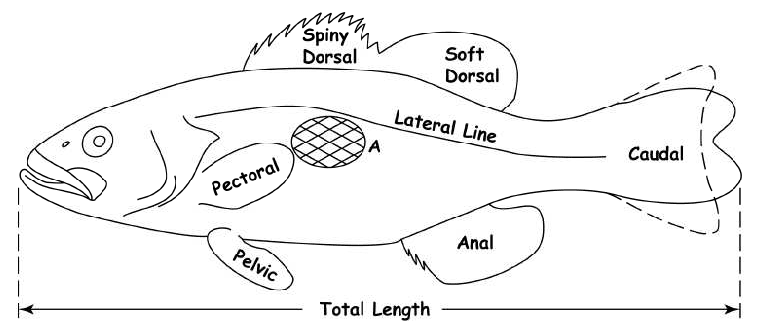
Length – Total length is defined as a straight-line measurement (not over the curve of the body) from the tip of the snout (with mouth closed) to the end of the caudal fin with the lobes squeezed together.
Weight – Total weight, accurately measured under good conditions.
Sex – Determine and record the sex when possible.
State of organs – This refers to sex organs. Record here whether the fish is immature or mature; and if mature, whether ripe or spent.
Gear – Record the method used in capturing the fish, such as gill net, trap net, seine, or angling.
Collector – Name of person who caught the fish.
Taking the Scale Sample
Age determination is easier if care is used when taking the scale sample. Scale samples should be taken from a definite area on the fish. The recommended location on spiny-rayed fishes is just below the lateral line and below the middle of the spiny dorsal fin.
For most soft-rayed fishes the area between the lateral line and the dorsal fin is preferred; for trout the best spot is directly below the lateral line beneath the posterior end of the dorsal fin
Making Age Determinations
To prepare scales for age determination, place four to six scales on a slide of clear plastic (vinyl or cellulose acetate, 0.5 mm thick) with sculptured side (side with ridges) down.
Then, sandwich the slide with the scales between two more pieces of plastic and run through a roller press, using enough pressure to make a distinct impression of the scales on the plastic slide.
Store the plastic slide with the scale impressions in the scale envelope from which the scales were taken. Only complete and normal scales can be used for age determinations. Abnormal or regenerated scales are often found on fish.
When a fish loses a scale, it grows a replacement lacking circuli and annuli in the center. Consequently, the early part of the growth history is lost.
To make age determinations (i.e., to “read” the scale), the plastic impression is viewed through a Micro-projector or microfiche reader that magnifies the impression up to 90 times, as needed.
Abinocular microscope provides suitable magnification for counting year marks, but if the scales are to be measured, as is done in “back calculation”, a micro-projector is needed.
Back-calculation
The back-calculation technique is useful for determining more precisely a fish’s growth during each year of life prior to the sampling date.
The results might reveal, for example, that a fish which is of average size for its age now, grew fast in certain earlier years and slow in other years.
The technique is especially useful if no growth samples were taken prior to a management activity or if only a few fish were sampled afterwards.
There are problems to be considered, however. Back-calculated lengths at age 1 and age 2 are imprecise if small fish were not sampled adequately.
Generally, it is not wise to extrapolate the fish length vs. scale radius relationship beyond the sizes actually sampled.
Another problem is “Lee’s phenomenon”. This is the tendency for the computed lengths of the older fish in their early years of life to be systematically lower than those of younger fish at the same age.
Read Also : Integrated Pest Management (IPM) in the Control of Storage Insects
That is, it appearsthat the slower-growing fish live the longest. This error can be minimized by sampling a wide range of fish sizes.
The procedure for back calculation is as follows:
Obtain scale samples from the same area of each fish. Ideally, use key scales (identical area) because they have the same shape.
While projecting the scale and counting annuli, measure with a ruler the radius of the scale and the distance to each annulus. Select a standard axis for measuring along (such as the axis from the focus to the middle of the anterior field) and use the same magnification for all samples in the collection.
Compute the relationship between fish length (L) and scale radius (S). This linear equation will usually give a satisfactory fit:
L = a + bS
Compute the length at each annulus (Ln) from the distance from the focus to that annulus (Sn).The process may be automated by projecting the scale image onto a digitizing pad or video monitor linked to a computer and “marking” each annulus with an electronic mouse or stylus.
Available software will then perform all the computations.
The intercept (a), also called the correction factor, is a very important parameter that is difficult to estimate. It may be thought of as the length at which scales begin to form, but in a practical vein it just helps make the data fit mathematically. The intercept should be determined for each species and each population.
Age Determination from the Otoliths
The otoliths (ear stones) are calcium carbonates materials, which are found in the brain cavity of fish and function as balancing organs. The use of otoliths is very important especially in fish, which are entirely scale less.
However, it is also paramount to note that age determination from otoliths can be disadvantageous, where valuable sport or commercial fish are concerned, because the fish have to be killed.
In the determination of age of fish from the otoliths, it is necessary to establish the following:
A recognizable pattern of rings or markings which can be visible in the otoliths by viewing directly in ordinary light of after some methods of preparation or staining
A regular time scale can be allocated to the visible pattern, this time scale is not necessary annual, although it is usually so, particularly in temperate regions. This is the essential part for validating the otoliths for age determination.
The otoliths have two distinguishable ring patterns: opaque and hyaline zones. The opaque zone is associated with period of fast growth while the hyaline zone is associated with period of slow growth.
Techniques for collection and preserving the otoliths
To obtain the otoliths, a dissection of the head is necessary. The otoliths are collected from under the brain tissue. After collection, they are either processed or viewed fresh or are preserved for later processing or viewing.
The otoliths can be preserved dry in paper envelopes or suitable vial after been rubbed between the fingers to remove mucus and adhering tissue. The use of formalin to preserve otoliths is prohibited.
It is impossible to determine age from bones of rotten fish or fish which have lain in formalin because such bones usually become discolored and decalcified.
Sometimes the otoliths may be rinsed in water and etched in 0.1% Hcl for about 5 minutes, follow by dehydration in alcohol.
Examination and Interpretation of Growth Marks on Otoliths
There are several ways to examine and interpret otoliths from many fishes:
Otolths from many fishes especially fresh water fishes can be examined adequately without modifications. Such otoliths is placed in water in a black dish and illuminated at an angle from above.
Against this background, the opaque zone appears as white rings while the hyaline zone appears as dark rings. To enhance the appearance of the growth rings the otoliths can be examined in oil such as glycerine, cedar or cresol.
If the otolith is thin, it can be examined directly without a dark background. Light is shone through the otolith as it is examined. Opaque zone appears as dark rings while the hyaline zone appears as white or dark rings, because the colors of these two zones are interchangeable depending on how it is illuminated, the term opaque and hyaline zones have to be used.
If the otoliths is old and compact, one surface can be graded first to make the growth mark more visible. Alternatively the otoliths can be broken into two between the thumbs but the cut surface must pass through the nucleus of the otoliths.
Then, the cut surface is then grinded flat. Grinding of otoliths is done with wet carborundum powder on a glass tile or a grinding stone (electrically or mechanically operated). After grinding the otolith is examined under the microscope, the opaque zone appears as dark rings while the hyaline zone appears white.
Burnt otoliths technique: the otoliths of some fish species are clearer when burnt on a low flame spirit. This is applied under gauze on which the otoliths is placed. The whole otoliths or burnt surface after grinding.
Scanning for Photo electric Measurement: The otoliths is scanned and the transparency of the two zones are recorded automatically on a recorder and the annuli become visible peaks on a slide.
Bones Method
Opercula, vertebrae, fin-rays and spines are the most frequently used. Except for the fin-rays and spines, collection of bones involves sacrificing the fish. Fish is dissected and the appropriate bone removed, clean of flesh and fats and store dry in a dry paper envelope.
The growth marks are best examined under a projecting microscope; the opaque zones are broad and white while the hyaline zone is narrow and dark.
Validation of Ageing Techniques
Since attempts at ageing of fish began, there has been a need to validate whether the growth increments seen are in fact annual in nature or follow some other cycle.
Most validation studies have involved injection of tagged fish with a chemical dye (typically oxytetracycline) that then penetrates the bony structures of the fish shortly after injection. These fishes are then released and if recaptured at a later date, the time period between when the chemical mark was laid down and the edge of the bony structure is known.
When the bony structures from the fish are sectioned and viewed using ultraviolet light the chemically induced band fluoresces.(Cappoetal.,2000).
The process of estimating fish age incorporates a procedural error associated with the structure being examined and an interpretational error due to the element of subjectivity inherent in all age estimations (Campanas, 2001).
For this reason, methods of age validation and estimation of aging precision have been developed. A variety of methods exist through which age interpretations can be validated. The methods described in table 1 (Campana, 2001) are the best available for insuring ageing accuracy.
Table 1: Features, advantages and disadvantages of methods used to confirm the accuracy of age interpretations. Methods are listed in descending order of scientific value.
Growth structure refers to either annulus (A) or daily growth increment (D), depending on application. Methods for age corroboration (such as length frequency analysis and tag-recapture analysis) are not shown here, but are described in detail in Campana (2001).
| Mark-recapture | AD | all | validates | number of | ± 1 yr | > 1 | > 1-10 | minimal |
| of chemically- | periodicity | recaptures of | yr | excluding | ||||
| tagged wild fish | of post- | fish at liberty | cost of | |||||
| tagging | more than 1 | tagging | ||||||
| growth | yr can be | cruise | ||||||
| structures | low or non- | |||||||
| in fish of | existent | |||||||
| any age | identification | |||||||
| of a single | ||||||||
| post-mark | ||||||||
| annulus can | ||||||||
| be |
| Radio chemical dating | A | 5+ yr | validates absolute age can be applied to any recently- collected samples well suited to long- lived fishes | can only distinguish between widely divergent age estimates | ±25-50% | 10-50 | < 1 yr | ~$1000 per age category |
| Progression of discrete length modes sampled for age structures | AD | 0-5 yr | well suited for validating the first 1-2 age classes | length mode must not overlap that of adjacent mode assumes no size- selective immigration or emigration into the sampling area | ± 0 yr | > 100 | 1 yr | minimal other than fish collection |
| Capture of wild fish with natural, date-specific markers | AD | all | validates periodicity of growth increments and sometimes absolute age | natural, date- specific markers are very rare | ± 0 yr | > 1 | > 1 yr | Minimal |
| Marginal increment analysis | A | all | validates periodicity of growth increments | only suited to fast- growing and/or young fish requires samples from throughout year | ± 1 yr | > 100 | 1 yr | minimal other than fish collection |
| Captive rearing from hatch | AD | all | validates both absolute age and periodicity of growth structures | otoliths increments in reared fish seldom resemble those of wild fish | ± 0 yr | > 1 | 1-10 yr | Minimal |
| Captive rearing of chemically- tagged fish | AD | all | validates periodicity of growth increments particularly easy for daily increments | otoliths increments in reared fish seldom resemble those of wild fish | ± 0 yr | > 1 | 1-10 yr | Minimal |
In summary, the most commonly used method for age determination is examination of markings which appears on the hard parts of fishes, These markings may be annual of formed during period of alternate fast and slow growth, usually caused by environmental, physiological and nutrition fluctuations.
Fish age and growth are critical correlates with which to evaluate many other biological; their studies provide information on stock composition, age at maturity of a fish, life span, mortality and production rate etc.
Direct estimate, anatomical method and length-frequency distribution methods are the basic methods of age determination; these are validated to know whether the growth increments seen are in fact annual in nature or follow some other cycle.
Read Also : Grey Water Complete Management Guide
