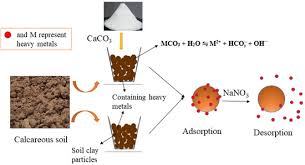
The three states of matter (solid, liquid, and gaseous phases) could interrelate with each other without having one of the phases consumed by the other phase. Water could be held on the surface of a solid, while gases could also be held on soil material.
The same applies to dissolved soil nutrients and soil colloids. The nutrients in solution are held on the surface of colloids because of the differing charges (positive and negative charges).
This surface phenomenon is called adsorption; this is opposed to absorption, where an entity penetrates the inner surfaces of the other phase.
The release of adsorbed materials is called desorption. In this article, readers will learn about the meaning of the concept of adsorption/desorption and the forces involved in the process.
Read Also: How to Grow, Use and Care for Whiteedge Flatsedge Grass (Cyperus flavicomus)
Adsorption and Desorption in Soil Nutrient Dynamics
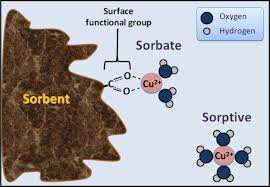
Adsorption is the process of concentrating materials at the interface of two phases. It may or may not lead to absorption, which is the penetration of a component into the material. When a distinction may not be made between these two processes, the term sorption is used.
Adsorption may occur either on the surface of a liquid or solid by a gas or liquid. Desorption is used to indicate the release or removal of materials that were adsorbed. The substance sorbed is called the sorbate, and the material in which sorption occurs is called the sorbent.
Adsorption Systems in Soil Chemistry
- Stationary phase: solid
- System: liquid – solid, gas – liquid, gas – solid
- Mobile phase: liquid, gas
- Adsorption: liquid – solid, gas – liquid, gas – solid
Characteristics of Adsorption in Agricultural Soils
Adsorption is dependent not only on the surface charge but also on the surface area. The amount of material adsorbed is directly proportional to the specific surface.
Adsorption reactions are reversible and are equilibrium reactions, i.e., adsorption and desorption are reversible. Adsorption is characterized by a positive heat of adsorption, meaning that energy is released during the adsorption process.
Adsorption generally decreases as temperature increases, i.e., adsorption is less at high temperatures. This is caused by an increased kinetic energy of the molecules at higher temperatures, which interferes with the concentrating process.
In contrast, the rate of a real chemical reaction increases as temperature is increased. Therefore, these differences can be used to distinguish an adsorption process from a true chemical reaction, although a similar equilibrium can be reached.
Read Also: How To Grow, Use and Care For Threeawn Grass (Aristida Spp.)
Forces Driving Adsorption in Soil Systems
Forces responsible for adsorption reactions include:
- Physical forces
- Hydrogen bonding
- Hydrophobic bonding
- Electrostatic bonding
- Coordination reaction
- Ligand exchange
Role of Adsorption and Desorption in Soil Fertility
Adsorption and desorption are reversible reactions. Cations are adsorbed on soil colloids and, hence, made available to plant roots when needed.
Desorption, on the other hand, is the detachment of the adsorbed materials from the colloidal complex. Adsorption and desorption are reversible reactions in the soil; they are dependent on several forces in the soil.
Do you have any questions, suggestions, or contributions? If so, please feel free to use the comment box below to share your thoughts. We also encourage you to kindly share this information with others who might benefit from it. Since we can’t reach everyone at once, we truly appreciate your help in spreading the word. Thank you so much for your support and for sharing!