Next to nitrogen in importance to plant nutrition is phosphorus and potassium, and their availability is dependent on many soil and other extraneous factors.
However, these nutrients are major nutrients needed in large quantities by plants. In this article, readers will learn about the factors that affect the ability of the soil to supply phosphorus (P) and potassium (K).
Phosphorus Availability in Agricultural Soils
1. Influence of Clay Content on Phosphorus Availability
Most of the compounds with which P reacts are in finer soil fractions. Therefore, if soils with similar pH values and mineralogy are compared, P fixation tends to be more pronounced, and ease of P release tends to be lowest in those soils with higher clay contents.
2. Impact of Clay Mineral Types on Phosphorus Retention
Generally, clays with greater anion exchange capacity (due to positive surface charge) have a greater affinity for phosphate ions. For example, high P fixation is characteristic of allophane clays found in Andisols and others with volcanic ash.
Oxides of Fe and Al, such as gibbsite and goethite, also strongly hold P. Among the layer silicates, kaolinite has a greater P-fixation capacity than others. The soil components responsible for P-fixing capacity are, in order of increasing extent and degree of fixation:
2:1 clay << 1:1 clays < carbonate crystals < crystalline Al, Fe, Mn oxides < amorphous Al, Fe, Mn oxides, allophane. i.e., Vertisols & Mollisols < Ultisols & Oxisols < Andisols.
3. Role of Soil pH in Phosphorus Availability
The greatest degree of P fixation occurs at very low and very high soil pH. As pH increases from below 5 to about 6, the Fe and Al PO₃²⁻ become more soluble. Also, as pH drops from greater than 8 to below 6, CaPO₃ compounds increase in solubility. Thus, P fixation is at its lowest (plant availability is highest) when soil pH is between 6 and 7.
Read Also: Goats Farming Complete Practical Guide
4. Effect of Organic Matter on Phosphorus Dynamics

Organic matter has little capacity to strongly fix P ions. This is due to large humic molecules that adhere to the surfaces of clay and metal hydrous oxide particles, masking the P-fixation sites.
Secondly, organic acids are attracted to positive charges and hydroxyls on the surfaces of clays and hydrous oxides; these organic anions may compete with P ions for fixation sites.
Certain organic acids can entrap Al and Fe in stable organic complexes called chelates. Once chelated, these metals are unavailable for P ions in solution.
Read Also: Introduction to Ruminant Animals Production
Potassium Availability in Agricultural Soils
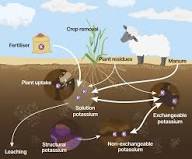
1. Influence of Clay Type and Soil Moisture on Potassium Retention
The ability of various soil colloids to fix K varies widely. Kaolinite and other 1:1 type clays fix little K. On the other hand, 2:1 clays, such as vermiculite, mica, and smectite, fix K very readily and in large quantities.
Alternating wetting/drying and freezing/thawing has been shown to enhance the fixation of K in non-exchangeable forms and release previously fixed K to the soil solution.
2. Impact of Soil pH on Potassium Fixation
Application of lime sometimes results in an increase in K fixation in soils. In acid soils, tightly held H⁺ and hydroxyl Al ions prevent K from being close to colloidal surfaces, which reduces its fixation.
As the pH increases, the H⁺ and hydroxyl Al ions are removed or neutralized, making it easier for K ions to move closer to the colloidal surfaces where they are more susceptible to fixation in 2:1 clays.
Soil clay type, pH, and organic matter are some of the major factors that determine the availability of P and K in the soil.
Phosphorus and potassium are important nutrients in the soil. Their availability is dependent on many soil factors.
Do you have any questions, suggestions, or contributions? If so, please feel free to use the comment box below to share your thoughts. We also encourage you to kindly share this information with others who might benefit from it. Since we can’t reach everyone at once, we truly appreciate your help in spreading the word. Thank you so much for your support and for sharing!

