Ecology is often referred to as the “study of distribution and abundance.” One of the first things a field ecologist will want to know about an animal or plant species is: How dense is the population units of density are the number of individuals or colonies, etc per unit area or volume. Another important question is: How are the organisms dispersed The pattern of distribution in space within the habitat?
In most cases, it is impossible to count every individual or plot their location on a map. This would be a census because of the time, effort, or money involved. So it would be useful if there were some way to get an accurate representation of some spatial characteristics of the population without having to map every organism.
By sampling the population, this can be done, but the sampling must be done properly if a valid representation is to be achieved. To ensure an adequate representation, some guidelines must be followed.
General Sampling Procedure
To obtain an unbiased estimate of the population, sampling should be done at random or more specifically, the sampling should be conducted in such a way that the probability of each individual being selected in the sample is the same.
There are several ways of ensuring this criterion is met – or at least approximated. Random numbers are series of numbers such that the chance of selecting, for example, any digit (0 – 9) is equal at any point in the sampling procedure. If the random numbers can be assigned to organisms or to locations in the habitat, they can be used to select the sample from the population.
One way to generate a series of random numbers is to write the numerals 0 through 9 on slips of paper, mix them in a hat, draw the slips out, write the number down, then replace the slip in the hat, remix, and draw again, etc.
A faster and less cumbersome method is to use a random number table. Random numbers can also be generated through calculators or spreadsheet applications, which can help in selecting sampling positions (e.g. paces along a trail, GIS coordinates, termite holes in a wall that have been numbered).
Read Also: Uses of cocoyam
Methods for Sampling Zooplankton

Three common methods for sampling zooplankton are net, trap, and tube. Nets are used most often, yet they have serious limitations regarding obtaining good quantitative data, especially in nutrient and algae-rich waters. Nets are conical devices made of fine nylon mesh that are pulled through the water either vertically or horizontally for a known distance.
Animals are captured in a vial or mesh-walled bucket at the bottom of the net and then can be rinsed into a storage bottle for counting.
The amount of water from which zooplankton are removed is estimated as the length of the tow times the mouth diameter of the net.
However, nets may not actually filter this volume of water. The main advantage of using a net is that samples of large volumes of lake water can be collected quickly. Nets can be obtained with various mesh sizes, depending on whether one wants to collect only the largest zooplankton or the entire size range that occurs in the water.
Trap Sampling Technique
The most common trap sampler is the Schindler-Patalas trap, named after the two scientists who invented the device. This is a clear plastic box that is lowered to a desired depth in the water column and then quickly closed (upper and lower doors) by pulling upward on the line by which the device is lowered and raised in the water.
This traps zooplankton inside the box. When lifted into the boat, the water is allowed to exit a small mesh net attached to the lower wall of the box, and zooplankton are collected inside a sampling bucket at the end of that net.
This device provides a high degree of certainty regarding the actual volume of water sampled, but if the water column is deep, many samples may be needed to collect animals from all depths from surface to bottom.
Tube Sampling Method
The third method is a tube, made of common PVC or Tygon. A tube is lowered into the water column, and when the bottom reaches the desired depth (near the sediments), a line is pulled to close the bottom with a rubber stopper or other device.
The tube is raised into the boat, and the collected water is poured through a net to collect the zooplankton. This device also provides a high degree of certainty about the volume of water sampled, but it may not be an effective way to sample large animals that occur at low densities or animals that can detect and escape from a narrow sampling device.
Nets, traps, and tubes will be used to collect representatives during the Zooplankton Ecology course, and students will participate in a critical analysis of these three common sampling techniques.
Counting and Biomass Estimation of Zooplankton

Simple counts of zooplankton can be done with a light microscope. For large zooplankton such as Daphnia, which occur at relatively low densities (1 to 100 per liter), the entire sample may be scanned at low magnification, counting all observed individuals.
For small zooplankton, such as rotifers and copepod nauplii, which occur at high densities (>1000 per liter), it is standard practice to count a known percentage of the sample volume at high magnification, and then multiply by total volume / counted volume to obtain the total number of animals in the sample.
Once the number of animals of each species in a sample is known, density in the lake is estimated as counts divided by the volume of water filtered with the net or collected by the trap or tube.
Quantitative Analysis of Plankton
The counting procedure generally involves recording the taxa observed and the number of algal units (objects) for each taxon in a known area of the counting chamber. Since the volume of sample added and area of the whole chamber observed is recorded, the concentration of each individual taxon can then be calculated.
Zooplankton Counting Procedure
The count should be carried out in the following manner: A low magnification (e.g. x40 or x100) whole chamber count to pick up large taxa, followed by transect counts at an intermediate magnification (x250), which are helpful to enumerate intermediate-sized taxa that are too small for the low-magnification count but too large to be reasonably counted using fields of view at high magnification.
A high magnification count (x400 or greater) using fields of view will pick up the small taxa. The goal is to count 100 fields of view (about 400 units, assuming the recommended sample concentration).
Read Also: The Complete Guide to Plantain Farming
Qualitative and Quantitative Evaluation of Plankton
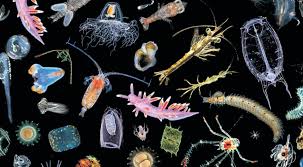
Replicate plankton samples, each of 50 liters, should be collected from various spots around a chosen river or lake by means of a bucket and filtered through a bolting silk plankton net of 50 μm. The filtrate should be transferred to another bottle and preserved immediately in 1:100 Lugol’s solution.
Qualitative and quantitative analysis of both phyto- and zooplankton should be done following the drop count method (APHA 1995). Identification of plankton is often made by following Ward and Whipple (1959) and Prescott (1962).
Oxygen Depletion in Wetland Soils and Anaerobic Conditions
The inundation or saturation of wetland soils by water leads to the formation of anaerobic conditions as oxygen is depleted faster than it can be replaced by diffusion. The rate of oxygen loss in flooded soils can vary depending on other soil conditions, such as temperature and rates of microbial respiration.
In most wetlands, small, oxidized layers of soil may persist on the surface or around the roots of vascular plants, but generally, anaerobic or reduced conditions prevail.
Freshwater Fisheries Ecology: Understanding Global Resources
Freshwater Fisheries Ecology defines what is available globally, what is at risk of being lost, and what can be mitigated. Given the right tools, much can still be saved. To estimate potential production, the dynamics of freshwater ecosystems (rivers, lakes, and estuaries) need to be understood.
These dynamics are diverse, as are the Earth’s freshwater fisheries resources (from boreal to tropical regions), and these influence how fisheries are both utilized and abused.
Do you have any questions, suggestions, or contributions? If so, please feel free to use the comment box below to share your thoughts. We also encourage you to kindly share this information with others who might benefit from it. Since we can’t reach everyone at once, we truly appreciate your help in spreading the word. Thank you so much for your support and for sharing!
Read Also: Complete Composting Guide for Beginners
Frequently Asked Questions
We will update this section soon.

