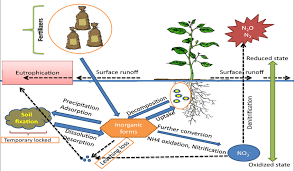
The nitrogen cycle shows soil nitrogen changes, inputs and losses, during the growth period of the plants as describe below.
Means by which Nitrogen is added to Soil-Plant System
1. Rainwater
There are atmospheric nitrogen compounds such as ammonia, nitrates, compounds released from the soil and plants, compounds from the soil and plants, and compounds from coal and petroleum industrial areas which are usually added to the soil through
rain.
Nitrogen inputs through rainfall are location-and season-dependent, usually greater around industrial cities, near large animal feed lots and areas with high annual precipitation such as the tropics.
The supply of N through rain fall may be up to 5 to 25kg/ha/year nitrogen, in a readily available form, thus assisting in the maintenance of soil fertility.
2. Nitrogen Fixation
The amount of dimolecular nitrogen in the air is about 2500 times larger than the global amount of combined nitrogen. This dinitrogen (N 2 ) has to be converted into forms useable by plants.
This change of N 2 gas to plant utilizable forms is known as Nitrogen fixation and it is carried out mainly by microorganisms in the soil in which case it is known as Biological N- fixation or through electric discharges (during lighting and thunder-storm) in which case the process is electric fixation. Chemical fixation occurs in the laboratory.
3. Electric Fixation
Nitrogen compounds such as nitrous oxide (N 2 0) and nitric axid (NO, NO x ) come mainly through electric discharge during lightening or thunderstorm.
The distribution of lightening over the globe is not equal being higher in the tropics than elsewhere. The principal form of NOx being from lightening is NO which has low solubility in water.
The nitrogen oxides have to be either in form of nitric acid or nitrogen dioxide (NO 2 ) to be easily removed from the atmosphere by precipitation.
There could be a small transformation rate from nitric oxide to form nitric acid (HNO 3 ) but that thunderstorm could be a substantial source of NOx and that the total quantity of nitrogen added to the soil is not insignificant.
4. Chemical Fixation
This process occurs in the laboratory whereby ammonia gas is formed from the elements hydrogen and nitrogen.
The process requires high temperature and pressures while it consumes large quantities of energy. Natural gas is the source of hydrogen and the N 2 comes from the atmosphere.
Several nitrogen compounds and fertilizer materials are then derived synthetically from ammonia. Some of these materials are nitrogen solutions, urea, ammonium nitrate, ammonium sulphates and ammonium phosphates.
The widths of the arrows roughly indicate the magnitude of the losses and the additions often encountered.
It should be emphasized that the diagram represents average conditions only and that much variability is to be expected in the actual and relative quantities of nitrogen involved. [from Brady and Weil (1999).
Read Also: Factors Affecting Mineralization and Nitrification Processes
5. Biological Nitrogen Fixation
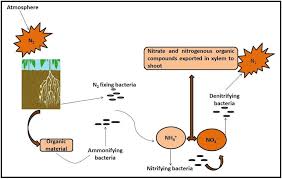
Biological nitrogen fixation is the conversion of dinitrogen (N 2) in soil air into combined forms useable by the plants and effected by microorganisms.
Some groups of microbes live freely in the soil where they are able to convert N 2 into body tissue nitrogen form and when they die and decompose, the combined nitrogen is released for plant use.
These groups of free living N-fixers are known as Non-symbiotic nitrogen fixing microorganisms. In the tropics Azotobacter and Bjeirinckia species are known to be free-living microbes that fix N 2 under aerobic well aerated soils of pH 6.0. Clostridium spp. Are free- living bacteria that fix N 2 under anaerobic conditions in soil.
Other- living soil micro-organisms that fix nitrogen are blue-green algae and Azospiriullum in the rhizosphere of certain plants. The range of fixed N by these group of non-symbiotic microbes is between 2-25 kg/ha/year.
In symbiotic fixation, bacterial and actinomycetes effect the formation of root nodules (abnormal root growth) in both legume and non legume plants and then inhabit those nodules where they fix nitrogen.
The host plant supplies the bacteria with carbohydrate as source of energy, and the bacteria supplies the plant with fixed
nitrogen compounds.in effect, both the plant and the microorganism have a mutually beneficial association known as symbiosis.
There are various species of Rhizobium bacteria that inhabit different plant species such as R. trifolic which inhabit clovers, R. japonicum for Glycine max (soya beans) and R. Phaseoli which associates with Phaseolus vulgaris (dry beans). Appropriate Rhizobia cultures are used to inoculate the soil for a particular crop to nodulate for the fixation of nitrogen.
The release of ammonium-nitrogen from soil organic matter decomposition by heterotropic soil organisms through series of enzymatic digestion of complex protein compounds is known as mineralization of nitrogen. That is, organic – N is converted to usable mineral – N. This is a major source of nitrogen in non-fertilized soils.
6. Nitrification of Ammonium
The mineralized ammonium ions have very short lifetime because it is rapidly oxidized to nitrate anions by bacteria in a two-step process called nitrification. First the ammonium is transformed to nitrite by Nitrosomonas bacteria and then to nitrate by Nitrobacter.
Fertilizer-N addition: Nitrogen fertilizers applied to crops supplement the quantity of nitrogen already present in the soil.