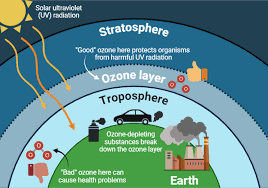
In this article, the topic of stratospheric ozone is studied. Its importance in absorbing ultraviolet radiation from the sun and protecting life on earth is explained.
The two regions of the atmosphere where ozone is found and the concept of the ozone hole are described. The article also explains what an ozone hole is and the series of processes that result in its formation. Efforts to reduce chlorofluorocarbons in the atmosphere are also discussed.
Understanding the Role of Stratospheric Ozone in Earth’s Atmosphere
Stratospheric ozone is important in the earth system because it absorbs ultraviolet radiation from the sun, protecting life on earth. Ozone is a relatively rare and unstable molecule composed of three oxygen atoms (O3).
The normal oxygen molecule has two oxygen atoms (O2). It is the second most common gas in the atmosphere and is relatively stable.
Ozone is found in two regions of the atmosphere:
- In the stratosphere at heights around 20–30 km, where it is produced by sunlight. This is good ozone. It is critical for life because it protects all life on earth from dangerous solar ultraviolet radiation, especially UVB, a band of ultraviolet radiation with wavelengths from 280–320 nanometers produced by the sun.
Ultraviolet radiation with wavelengths from 320–400 nanometers (UVA) is not absorbed and is much less dangerous to life.
- Close to the surface, where it is produced by sunlight acting on atmospheric pollutants. It is produced from nitrogen oxides and volatile carbon-based compounds when there is intense sunshine, especially in spring and summer. This is bad ozone. It causes respiratory illness, damages plants, and attacks rubber.
Read Also: The Yams Pistil: Economic Importance, Uses, and By-Products
Ozone Production and Destruction Processes in the Stratosphere
Ozone concentration in the stratosphere is due to a balance between production and destruction. Here is a somewhat simplified overview of key reactions known up to 1984, leaving out many other possible chemical reactions in the stratosphere.
1. Production: Ultraviolet (UV) radiation from the sun splits molecules O2 into two free oxygen atoms (O), which immediately combine with oxygen to produce ozone (O3) with the help of a random air molecule M (N2 or O2).
i. Eq. (1) O2 + uv-light → 2 O
ii. Eq. (2) 2 O + 2 O2 + M → 2 O3 + M
Production is greatest high in the tropical atmosphere at heights near 40 km. Circulation in the stratosphere then carries the ozone to other regions.
2. Destruction: Solar radiation of any wavelength from near-infrared to ultraviolet can destroy ozone. This destruction is greatest high in the tropical atmosphere at heights near 40 km.
i. Eq. (3) O3 + sunlight → O2 + O
ii. Eq. (4) O + O + M → O2 + M
iii. Eq. (5) O + O3 → 2 O2
This reaction is relatively weak because almost all the O atoms combine with molecular oxygen to remake ozone (Eq. 2). These interactions among O, O2, and O3 are called the Chapman reactions, described by Sydney Chapman in 1929 and 1930.
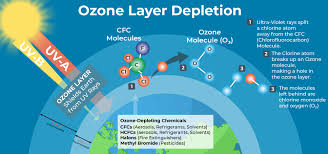
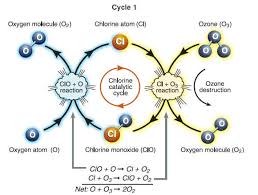
Chlorine Catalyzed Ozone Destruction in the Stratosphere
One chlorine atom can catalyze the destruction of about 100,000 ozone molecules before becoming incorporated into inert molecules such as HCl (hydrochloric acid) and ClONO2 (chlorine nitrate) through reactions:
i. Eq. (8) Cl + CH4 → CH3 + HCl
ii. Eq. (9) OH + ClO → O2 + HCl
iii. Eq. (10) H2O + Cl → O2 + HCl
iv. Eq. (11) ClO + NO2 + M → ClONO2 + M
Both HCl and ClONO2 are relatively stable and remain in the air. Their concentrations gradually increase as CFCs are destroyed by sunlight. Winds eventually carry HCl into the troposphere, where it is rained out.
This is the primary pathway for removing CFCs from the atmosphere: Transport to the stratosphere, conversion of fluorine and chlorine to acids (HF and HCl), transport of the acids to the troposphere, and removal of HF and HCl from the troposphere by precipitation.
There are two types of chlorine molecules in the stratosphere:
i. Active, ozone-destroying molecules
ii. Non-ozone destroying molecules: HCl and ClONO2
Read Also: The Soybean Epicotyl: Economic Importance, Uses, and By-Products
Scientific Discoveries on Ozone Destruction by Chlorine
Three important studies advanced understanding:
1. Paul Crutzen (1970): Showed that NO and NO2 are catalysts able to destroy ozone.
2. Richard Stolarski and Ralph Cicerone (1973): Demonstrated that chlorine is even more effective than nitrogen oxides in destroying stratospheric ozone.
3. Mario Molina and John Rowland (1974): Showed that man-made chlorofluorocarbon (CFC) gases are the most important source of free chlorine in the stratosphere, causing significant reductions in ozone levels.
CFCs have long lifetimes (50–500 years) because they do not dissolve in water and are chemically inert. They are broken down mostly in the stratosphere by ultraviolet radiation, releasing chlorine atoms.
Understanding the Ozone Hole Over Antarctica
Ozone concentration in the stratosphere over Antarctica in the southern hemisphere during spring has become much less compared to the period up to 1980. This large area of low ozone concentration is called the ozone hole.
The first measurements of ozone concentration above Antarctica were made by the British Antarctic Survey at Halley Bay starting in 1965. Global measurements began in 1978 with the launch of the Total Ozone Mapping Spectrometer (TOMS) on Nimbus-7.
Scientists first noticed that ozone over Antarctica was slowly decreasing during the 1960s. Joseph Farman, Brian Gardiner, and Jonathan Shanklin measured extraordinarily low ozone in October 1984 during the Antarctic spring and published their findings in May 1985. The very low ozone measurements in 1984 were the first clear indication of an ozone hole above Antarctica.
Since then, ozone values continued to decrease, and the area of the ozone hole expanded from about 5 million square kilometers in 1984 to 28 million square kilometers in 2006. For comparison, 24 million square kilometers is about the size of North America.
Explaining the Antarctica Ozone Hole Theory
The reactions described by Chapman, Crutzen, Molina, and Rowland (Eq. 1 to Eq. 11) cannot explain the size of the ozone hole. They predict only a small global reduction of ozone.
Field experiments flying instruments on airplanes in the Antarctic stratosphere and new satellite measurements answered important questions.
Processes Leading to the Ozone Hole Formation
During winter, very cold air over Antarctica is surrounded by warmer air at lower latitudes. This creates a low-pressure region with strong winds blowing around the region at the boundary between warm and cold air. The rotating air, known as the strong polar vortex, isolates the stratosphere above Antarctica.
As the air cools, Polar Stratospheric Clouds (PSCs) form inside the vortex:
i. At 195 K, nitric acid, sulfuric acid, and water condense to form Type I PSCs.
ii. At 188 K, H2O molecules condense on Type I cloud particles to form Type II PSCs.
Type II particles are large enough (10 microns in diameter) to fall out of the stratosphere, removing nitric acid and water. Type I particles are smaller (1 micron in diameter) and remain suspended.
Polar stratospheric clouds are important because:
- Chlorine nitrate (ClONO2) and hydrochloric acid (HCl) molecules attach to cloud particles. Chemical reactions on the surface convert non-ozone destroying molecules (HCl and ClONO2) into Cl2, building up a reservoir of Cl2 during the winter.
Important reactions include:
i. Eq. (12) HCl + ClONO2 → Cl2 + HNO3
ii. Eq. (13) ClONO2 + H2O → HOCl + HNO3
iii. Eq. (14) HCl + HOCl → H2O + Cl2
The nitric acid becomes incorporated into the cloud particles.
In addition:
i. Nitrogen oxides condense to form clouds.
ii. Other nitrogen oxides react with H2O to produce nitric acid.
iii. Cloud particles fall out, removing nitric acid (HNO3) and other nitrogen-containing molecules.
In spring, the first sunlight warms cloud particles, releasing Cl2. Ultraviolet light splits Cl2 into two chlorine atoms, initiating ozone-destroying reactions and leading to the ozone hole.
Global Efforts to Reduce Chlorofluorocarbons (CFCs)
The work by Mario Molina and John Rowland in 1974 convinced the U.S. and other governments to ban the use of CFCs as a propellant in aerosol cans by 1977. Legislation was easily passed because other gases could substitute for CFCs.
The discovery of the Antarctic ozone hole in 1985 showed the destruction of ozone was much worse than expected. This led to an international effort to ban the production and use of CFCs.
The Montreal Protocol of 1987 and later amendments at meetings in London (1990), Copenhagen (1992), Montreal (1997), and Beijing (1999) banned the manufacture of most ozone-depleting gases.
Final Notes on the Importance of Stratospheric Ozone for Agriculture
Stratospheric ozone is critical in the earth system because it absorbs ultraviolet radiation from the sun, protecting life on earth. Ozone is a relatively rare and unstable molecule composed of three (3) oxygen atoms (O3).
The concept of the ozone hole was explained, and efforts to reduce chlorofluorocarbons in the atmosphere were discussed.
The article elaborated on the concept of stratospheric ozone, emphasizing its importance to life on planet Earth. The discovery of the ozone hole in 1985 showed the destruction of ozone was much worse than expected.
Efforts to reduce chlorofluorocarbons (one of the greenhouse gases) in the atmosphere should therefore be intensified to protect agricultural productivity and environmental sustainability.
Do you have any questions, suggestions, or contributions? If so, please feel free to use the comment box below to share your thoughts. We also encourage you to kindly share this information with others who might benefit from it. Since we can’t reach everyone at once, we truly appreciate your help in spreading the word. Thank you so much for your support and for sharing!