Radioactivity is employed for biological research as radioisotopes have been used to label the test molecules resulting in effective and safe means of monitoring molecular interactions. It is the only technique that gives accuracy and specificity to radioactive isotopes/tracers.
The properties of radioisotopes that favor their use in biological analysis is that individual radioactive decay or emission has its own unique and specific energy.
What is Radioisotope?
Radioisotopes are elements that emit some extraordinary types of energy in the form of rays (alpha, beta, and gamma), which are very helpful to agricultural activities among other usefulness for human beings, in minute quantity. The rays emitted by radioisotopes are not visible to human eyes but can penetrate solid materials such as skin and eyes.
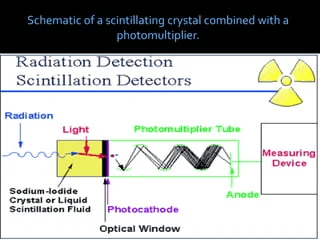
An instrument such as Geiger Muller and Scintillation Counters are normally used for detecting such invisible energy. These instruments are designed to detect the minutest quantity of this element.
The Use of Radioisotopes In Agriculture
It is commonly used in the field of agriculture than any other science field.
Nutritional Studies
In agriculture, radioisotopes are used in the nutritional studies of major and minor elements, milk production, and mechanism of photosynthesis studies. Radioisotopes are also important to the study and research in plant protection, plant pathology, action of insecticides, uptake of fertilizers, ions mobility in soil, and plants and food preservation.
It is also used to determine the correct nutrition for a plant we need to know the exact soil-plant relationship and the factors involved therein.
Read Also Agribusiness and Supply Chain Management
Advantages of Radioisotopes to Agriculture
1. With the help of radioisotopes, we can easily locate the presence of a single atom and molecule and their movement.
2. Hence, they allow research workers to follow up step by step on all kinds of processes that are related to the nutrition of plants from germination to maturity.
3. Very small quantities of labeled nutrients can be accurately measured in the presence of large quantities of other nutrients.
4. The location of materials can be identified by radio-autography.
5. The tracer technique enables one to trace those elements taken by the plants accurately and precisely.
6. It also helps to study accurately the effect of one element upon the absorption of another and their interaction by plants and now it has become very easy to study properly the phenomenon of interaction among the mineral nutrients.
7. They are used for soil analysis, especially in detecting the P content of the soil.
8. Radioactive phosphorus is normally used by researchers to distinguish between soil phosphorus and fertilizer phosphorus, taken up by plants.
9. Many elements are leached through the soil. Radioisotopes like Fe, Mn, K, Ca, N, Rb, C, Cs, Si and Sr, etc., and other macro and micro-elements have also been used by researchers to find out how these elements move in different types of soils and also their position in different clay fractions of the soils.
10. The radioisotope method is very reliable and helpful in the determination of soil fertility.
11. The Radioisotopes technique is used to determine the amount of nutrients that are taken by plants. It also helps to know the movement and the places of accumulation of various elements in the plant.
12. Radioisotopes are very useful in genetic studies. This is because they are used for mutation induction. The mutation is a sudden heritable change of the hereditary factors located in the DNA found in the chromosomes of the organisms, plant or animal.
Read Also Methods of Evaluation of Animal Body Composition
Tracer Technique
It is a technique in which one or more atoms of a chemical compound have been replaced by a radioisotope to trace the metabolic pathways. A radioactive tracer can also be used to track the distribution of a substance within a natural system such as a cell or tissue.
Radioactive tracers form the basis of a variety of imaging systems, such as PET scans, SPECT scans, and technetium scans. It is based on the principle that a stable isotope is replaced by a radioisotope. The radioisotope is capable of emitting radiations that can be detected and analyzed.
It is more powerful than chemical reactions as they can be detected even in lower concentrations as seen inside a cell. In general, isotopes of hydrogen, carbon, phosphorus, sulfur, and iodine have been used extensively to trace the path of biochemical reactions.
In metabolism research, Tritium(3H) and 14C-labeled glucose are commonly used to measure rates of glucose uptake, fatty acid synthesis, and other metabolic processes.
Autoradiography
The common application of radioisotopy is the use of autoradiography in combination with gel electrophoresis or microscopy. Autoradiography of gels is used to identify and quantify specific proteins or nucleic acids. Radioactive markers could be localized to particular cells in specific parts of the tissues or within cells using autoradiography.
The protocol for autoradiography is as follows;
1. Fix tissue or cells to slide. This is done after radioactive isotopes have been incorporated into cellular molecules.
2. Cover with a photographic emulsion. Ionizing radiations should be emitted during radioactive decay as the silver ions in the photographic emulsion become reduced to metallic silver grains.
3. Develop the emulsion after sufficient exposure
4. Examine the slide under the microscope. It will be observed that dark grains (or spots) will appear over the cells or structures that contain the radioactive marker.
5. These grains do not only detect radioactivity but provide information concerning the quantity and cellular distribution of radioactive labels (due to their number and distribution)
Principles and Application Of Manometry
1. Manometry
The technique of measuring the pressure variation due to gas production and consumption caused by biochemical reactions or physical changes. The manometer is the name of the instrument that measures and compares the pressure of the fluid.
Manometry applies to any bioprocess that involves the production/consumption of a poorly soluble gas. The main applications of manometry include: Aerobic processes (oxygen consumption) anoxic processes (N2 production) anaerobic processes (biogas production)
2. Manometer
The instrument consists of a glass U-tube such that if pressure is exerted on one end of the U- shaped tube (partially filled with liquid), the liquid will be displaced upwards on the other side of the tube.
The difference in height or distance between the levels of these two arms is proportional to the difference in pressure difference on each side of the tube.
3. Microcalorimetry
Calorimetry is the technique of measuring the heat produced by chemical reactions or physical changes. A microcalorimeter is an instrument that can measure really small heat exchange (5-‐10 mW L-‐1). It is sensitive up to 0.001°C which is equivalent to 3-‐5 mg BCOD L-‐1.
It can measure any temperature change associated with biochemical reactions. It is the universal tool for the study, optimization, and modeling of bioprocesses (substrate consumption as well as biomass adaptation, inhibition, and growth).
In conclusion, there are safe and effective means of quantifying and identifying specific proteins in cells/tissues of animals such as the use of radioisotopy. Pressure in fluids can be effectively determined using the manometry technique. Radioisotope is an effective and safe way of monitoring molecule interaction.
Specific proteins can be identified and quantified by the radioisotopy technique. Manometry measures pressure while the Claorimetry measures heat.