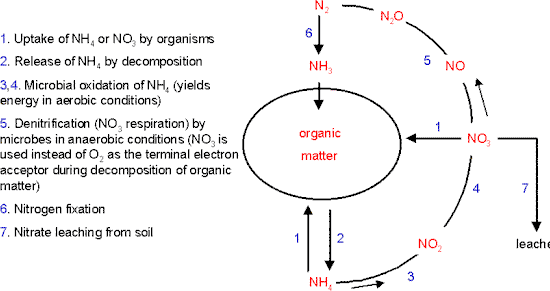
The diagram below shows an overview of the nitrogen cycle in soil or aquatic environments. At any one time a large proportion of the total fixed nitrogen will be locked up in the biomass or in the dead remains of organisms (shown collectively as “organic matter”).
So, the only nitrogen available to support new growth will be that which is supplied by nitrogen fixation from the atmosphere (pathway 6 in the diagram) or by the release of ammonium or simple organic nitrogen compounds through the decomposition of organic matter (pathway 2).
Some of other stages in this cycle are mediated by specialized groups of microorganisms and are explained below.
Nitrification
The term nitrification refers to the conversion of ammonium to nitrate (pathway 3-4). This is brought about by the nitrifying bacteria, which are specialised to gain their energy by oxidising ammonium, while using CO2 as their source of carbon to synthesise organic compounds.
Organisms of this sort are termed chemoautotrophs – they gain their energy by chemical oxidations (chemo-) and they are autotrophs (self- feeders) because they do not depend on pre-formed organic matter.
In principle the oxidation of ammonium by these bacteria is no different from the way in which humans gain energy by oxidising sugars. Their use of CO2 to produce organic matter is no different in principle from the behaviour of plants.
Read Also : Nitrogen Fixation and Role of Nitrogenin the Biosphere
The nitrifying bacteria are found in most soils and waters of moderate pH, but are not active in highly acidic soils.
They almost always are found as mixed-species communities (termed consortia) because some of them e.g. Nitrosomonas species are specialized to convert ammonium to nitrite (NO2–) while others e.g. Nitrobacterspecies – convert nitrite to nitrate (NO3–).
In fact, the accumulation of nitrite inhibits Nitrosomonas, so it depends on Nitrobacter to convert this to nitrate, whereasNitrobacterdepends on Nitrosomonas to generate nitrite.
The nitrifying bacteria have some important environmental consequences, because they are so common that most of the ammonium in oxygenated soil or natural waters is readily converted to nitrate.
Most plants and microorganisms can take up either nitrate or ammonium (arrows marked “1” in the diagram). However, process of nitrification has some undesirable consequences.
The ammonium ion (NH4+) has a positive charge and so is readily adsorbed onto the negatively charged clay colloids and soil organic matter, preventing it from being washed out of the soil by rainfall.
In contrast, the negatively charged nitrate ion is not held on soil particles and so can be washed down the soil profile – the process termed leaching (arrow marked 7 in the diagram).
In this way, valuable nitrogen can be lost from the soil, reducing the soil fertility. The nitrates can then accumulate in groundwater, and ultimately in drinking water.
There are strict regulations governing the amount of nitrate that can be present in drinking water, because nitrates can be reduced to highly reactive nitrites by microorganisms in the anaerobic conditions of the gut.
Nitrites are absorbed from the gut and bind to haemoglobin, reducing its oxygen-carrying capacity. In young babies this can lead to respiratory distress – the condition known as “blue baby syndrome”. Nitrite in the gut also can react with amino compounds, forming highly carcinogenic nitrosamines.
Read Also : Waste Management, Types and Classification of Wastes