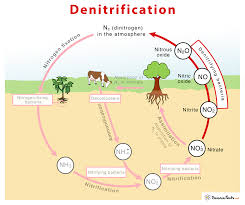
Denitrification refers to the process in which nitrate is converted to gaseous compounds (nitric oxide, nitrous oxide and N2) by microorganisms. The sequence usually involves the production of nitrite (NO2-) as an intermediate step is shown as “5” in the diagram above.
Several types of bacteria perform this conversion when growing on organic matter in anaerobic conditions. Because of the lack of oxygen for normal aerobic respiration, they use nitrate in place of oxygen as the terminal electron acceptor. This is termed anaerobic respiration and can be illustrated as follows:
In aerobic respiration (as in humans), organic molecules are oxidised to obtain energy, while oxygen is reduced to water:
C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy
In the absence of oxygen, any reducible substance such as nitrate (NO3-) could serve the same role and be reduced to nitrite, nitric oxide, nitrous oxide or N2.
Thus, the conditions in which we find denitrifying organisms are characterised by (1) a supply of oxidisable organic matter, and (2) absence of oxygen but availability of reducible nitrogen sources.
A mixture of gaseous nitrogen products is often produced because of the stepwise use of nitrate, nitrite, nitric oxide and nitrous oxide as electron acceptors in anaerobic respiration.
The common denitrifying bacteria include several species of Pseudomonas, Alkaligenes and Bacillus. Their activities result in substantial losses of nitrogen into the atmosphere, roughly balancing the amount of nitrogen fixation that occurs each year.
Root Noodle Symbioses
Legume Family
Plants that contribute to nitrogen fixation include the legume family Fabaceae with taxa such as clovers, soybeans, alfalfa, lupines, peanuts, and rooibos. They contain symbiotic bacteria called Rhizobia within nodules in their root systems, producing nitrogen compounds that help the plant to grow and compete with other plants.
When the plant dies, the fixed nitrogen is released; making it available to other plants and this helps to fertilize the soil. The great majority of legumes have this association, but a few genera (e.g., Styphnolobium) do not.
In many traditional and organic farming practices, fields are rotated through various types of crops, which usually includes one consisting mainly or entirely of clover or buckwheat (non-legume family Polygonaceae), which are often referred to as “green manure.”
Inga alley farming relies on the leguminous genus, Inga a small tropical, tough- leaved, nitrogen-fixing tree.
Non-leguminous


Although by far the majority plants able to form nitrogen-fixing root nodules are in the legume family Fabaceae, there are a few exceptions:
Parasponia, a tropical Celtidaceae also able to interact with rhizobia and form nitrogen-fixing nodules
Actinorhizal plants such as alder and bayberry, that can also forms nitrogen- fixing nodules, thanks to a symbiotic association with Frankia bacteria. These plants belong to 25 genera distributed among 8 plant families.
The ability to fix nitrogen is far from universally present in these families. For instance, of 122 genus in the Rosaceae, only 4 genera are capable of fixing nitrogen.
Read Also : The Nitrogen Cycle and Nitrification Guide
All these families belong to the orders Cucurbitales, Fagales, and Rosales, which together with the Fabales form a clade of eurosids. In this clade, Fabales were the first lineage to branch off; thus, the ability to fix nitrogen may be plesiomorphic and subsequently lost in most descendants of the original nitrogen-fixing plant; however, it may be that the basic genetic and physiological requirements were present in an incipient state in the last common ancestors of all these plants, but only evolved to full function in some of them:
Coriariaceae: Coriaria Datiscaceae: Datisca Elaeagnaceae: Elaeagnus(silverberries) Hippophae(sea-buckthorns) Shepherdia(buffaloberries)……
There are also several nitrogen-fixing symbiotic associations that involve cyanobacteria (such as Nostoc):
Some lichens such as Lobariaand Peltigera
Mosquito fern (Azollaspecies)
Cycads
Gunnera
Chemical nitrogen fixation
Haber process
Nitrogen can also be artificially fixed as ammonia for use in fertilizers, explosives, or in other products. The most common method is the Haber process. Artificial fertilizer production is now the largest source of human- produced fixed nitrogen in the Earth’s ecosystem.
The Haber process requires high pressures (around 200 atm) and high temperatures (at least 400 °C), routine conditions for industrial catalysis. This highly efficient process uses natural gas as a hydrogen source and air as a nitrogen source.
Dinitrogen Complexes
Much research has been conducted on the discovery of catalysts for nitrogen fixation, often with the goal of reducing the energy required for this conversion.
However, such research has thus far failed to even approach the efficiency and ease of the Haber process. Many compounds react with atmospheric nitrogen under ambient conditions. For example, lithium metal converts to lithium nitride under an atmosphere of nitrogen. Treatment of the resulting nitride gives ammonia.
The first dinitrogen complex was reported in 1965 based on ammonia coordinated to ruthenium ([Ru(NH3)5(N2)]2+). Research in chemical fixation from then on focused on transition metal complexes.
Since then, a large number of transition metal compounds that contain dinitrogen as a legend have been discovered. The dinitrogen ligand can either be bound to a single metal or bridge two (or more) metals.
The coordination chemistry of dinitrogen is complex and currently under intense investigation. This research may lead to new ways of using dinitrogen in synthesis and on an industrial scale.
Ambient Nitrogen Reduction
Catalytic chemical nitrogen fixation at temperatures considerably lower than the Haber process is an ongoing scientific endeavor. Nitrogen was successfully converted to ammonia and hydrazine by Alexander E. Shilov in 1970. The first example of homolytic cleavage of dinitrogen under mild conditions was published in 1995.
Two equivalents of a molybdenum complex reacted with one equivalent of dinitrogen, creating a triple bonded MoN complex.] Since then, this triple bounded complex has been used to make nitriles.
The first catalytic system converting nitrogen to ammonia at room temperature and pressure was discovered in 2003 and is based on another molybdenum compound, a proton source, and a strong reducing agent. However, this catalytic reduction fixates only a few nitrogen molecules.
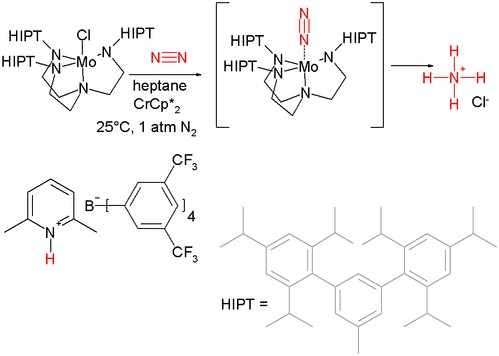
In 2011 Arashiba et al. reported another system with a catalyst again based on molybdenum but with a diphosphorus pincer ligand.
- Birkeland–Eyde process
- Haber process
- Denitrification
- George Washington Carver
- Nif gene
- Nitrification
- Nitrogen cycle
- Nitrogen deficiency
- Nitrogenase
- Push–pull technology
Read Also : Methods of Disposal of Waste Pesticide Containers
Frequently Asked Questions
We will update this section soon.