Proteins are a group of compounds containing carbon, hydrogen, oxygen, nitrogen (about 16%), and sulfur. In some proteins, phosphorus or iron is present, and occasionally, they may contain iodine, copper, and zinc. The approximate average elementary composition of proteins is as follows:
Element | Percentage
Carbon | 50%
Hydrogen | 7%
Oxygen | 23%
Nitrogen | 16%
Sulfur | 0-3%
Phosphorus | 0-3%
Other elements may be present in small amounts.
Proteins are found in all living cells of plants and animals, where they are intimately connected with all phases of cellular activity. Each species has its specific proteins, and a single organism contains many different proteins in its cells and tissues.
This results in a large variety of proteins occurring in nature. All proteins are composed of amino acids, with 20 standard amino acids forming their structure. The proteins differ in the number and sequence of these amino acids.
Physical and Chemical Properties of Proteins
1. Taste: Pure proteins are generally tasteless, though protein hydrolysates (proteoses, peptones, peptides, amino acids) often have a bitter taste.
2. Odor: Pure proteins are odorless. When heated, they turn brown, char, and release an odor similar to burning feathers or hair.
3. Molecular Weight: Proteins are large, complex molecules. Due to their size, they belong to the colloidal state of matter.
4. Solubility: All proteins exhibit colloidal properties and differ in water solubility. Some, like keratin, are insoluble, while others, like albumins, are highly soluble. Soluble proteins can be precipitated using salts such as sodium chloride or ammonium sulfate without altering their properties. They can be redissolved upon dilution.
5. Amphoteric Nature: Proteins contain free amino and carboxyl groups, making them amphoteric. They exhibit characteristic isoelectric points and buffering properties.
6. Denaturation: Proteins can be denatured or structurally altered by heat, strong acids, alkalis, alcohol, acetone, urea, or salts of heavy metals. Denaturation affects their chemical, physical, and biological properties. A common example is the coagulation of egg white when heated.
Read Also: Newcastle Disease: Symptoms and Prevention
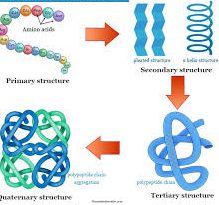
Structure Of Proteins
Proteins have four levels of structural organization:
1. Primary Structure: Proteins are formed by linking amino acids through peptide bonds between the α-carboxyl of one amino acid and the α-amino group of another. This sequence of amino acids along the polypeptide chain defines the protein’s primary structure.
2. Secondary Structure: The secondary structure refers to the folding of the amino acid chain due to hydrogen bonding between adjacent amino acids. This structure can exist as an α-helix, β-pleated sheet, or a random coil configuration.
3. Tertiary Structure: Tertiary structure describes the further folding and bending of the polypeptide chain due to interactions between the R groups of amino acid residues. This gives each protein its unique biological activity.
4. Quaternary Structure: Proteins with multiple polypeptide chains exhibit quaternary structure. These structures are stabilized by hydrogen bonds and electrostatic interactions between the chains.
Classification of Proteins
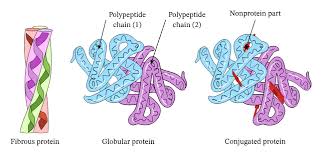
Proteins are classified based on physical and chemical properties. They are divided into three main categories: simple, conjugated, and derived proteins.
1. Simple Proteins
Simple proteins yield only amino acids or their derivatives upon hydrolysis.
i.Albumins: Soluble in water and coagulated by heat. Found in eggs (egg albumin), muscle (myosin), blood (serum albumin), milk (lactalbumin), peas (legumelin), and wheat (leucosin).
ii. Globulins: Insoluble in water but soluble in dilute salt solutions. Found in eggs (ovoglobulin), blood (serum globulin), beans (phaseolin), peas (legumelin), peanuts (arachin), and almonds (amandin).
iii. Glutelins: Soluble in dilute acids or alkalis but insoluble in neutral solvents. Found in wheat (gluten) and rice (oryzenin).
iv. Prolamins: Soluble in alcohol but insoluble in water and neutral solvents. Found in corn (zein), barley (hordein), and wheat (gleadin).
v. Albuminoids (Scleroproteins): Insoluble in water and resistant to acids and alkalis. Found in hair, horns, hooves (keratins), connective tissues (elastin), bones and tendons (collagen).
vi. Histones: Soluble in water and acids, insoluble in dilute ammonia, and not coagulated by heat. Example: globin in hemoglobin.
vii. Protamins: Simple polypeptides rich in basic amino acids. They form salt combinations with acids, mainly nucleic acids.
2. Conjugated (Complex) Proteins
These proteins contain a prosthetic (non-protein) group along with amino acids.
i. Nucleoproteins: Composed of simple proteins bound to nucleic acids. Found in yeast and glandular tissues.
ii. Mucoproteins (Mucoids): Combined with mucopolysaccharides. Found in connective tissues, egg white, and urine.
iii. Chromoproteins: Contain a colored prosthetic group. Examples include hemoglobin (iron-containing), cytochromes (cell oxidation), and flavoproteins (riboflavin-containing).
iv. Phosphoproteins: Contain phosphoric acid. Examples include casein (milk protein) and vitellin (egg yolk protein).
v. Lipoproteins: Combined with lipids such as lecithin and cephalin. Found in blood, milk, egg yolk, and plant chloroplasts.
vi. Metalloproteins: Contain metal ions like iron, zinc, copper, and magnesium. These metals are essential to their function.
Read Also: Popular Breeds of Ruminant Animals
Derived Proteins and Their Functional Diversity
Derived proteins are substances obtained from simple and conjugated proteins. This category is the least well-defined among protein groups. Derived proteins are further classified into primary derived proteins and secondary derived proteins based on the extent of modification.
1. Primary Derived Proteins
These proteins undergo slight modifications in their molecular structure and properties, without significant hydrolytic cleavage of peptide bonds. They are often referred to as denatured proteins.
i. Proteans: Insoluble products formed by the initial action of water, mild acids, or enzymes on globulins. They resemble glutelins in physical properties. Examples include myosan (from myosin), edestan (from edestin), and fibrin (from fibrinogen).
ii. Metaproteins: Formed by the further action of acids or alkalis on proteins. They are soluble in dilute acids and alkalis but insoluble in neutral solvents. Examples include acid and alkali albuminates.
iii. Coagulated Proteins: These are insoluble proteins produced by heat, alcohol, ultraviolet light, X-rays, or mechanical agitation at the isoelectric pH. Examples include cooked egg albumin, cooked meat proteins, and alcohol-precipitated proteins.
2. Secondary Derived Proteins
These result from the progressive hydrolytic cleavage of peptide bonds in proteins. The breakdown products are classified based on molecular complexity.
i. Proteoses (Albumoses): Soluble in water, not coagulated by heat, and precipitated by ammonium sulfate saturation.
ii. Peptones: Simpler than proteoses, soluble in water, not coagulated by heat, and precipitated by phosphotungstic acid.
iii. Peptides: Composed of a few amino acids linked by peptide bonds. Named based on the number of amino acids, such as dipeptides, tripeptides, and polypeptides. Peptides are water-soluble, not coagulated by heat, and often precipitated by phosphotungstic acid.
The complete breakdown of a protein into amino acids follows this sequence:
Protein → Protean → Metaproteins → Proteose → Peptone → Peptides → Amino Acids
Protein Denaturation
Most proteins function within a narrow range of temperature and pH. Exposure to extreme conditions causes denaturation, altering their structure and reducing solubility. Denaturation does not break peptide bonds, so the primary structure remains intact. Most globular proteins denature when heated above 60-70°C.
A common example is egg white coagulation when boiled. Denaturation can also result from alcohol, heavy metal salts, strong acids, alkalis, and trichloroacetic acid. This process usually leads to a loss of biological activity, such as the destruction of enzyme catalytic properties.
Functional Diversity of Proteins
Proteins play a wide range of biological roles in living organisms, especially in agriculture and food science.
1. Structural Role: Proteins provide the structural framework for tissues. Collagen forms connective tissues and bones, keratin is found in skin, feathers, and hooves, and elastin provides elasticity in tissues.
2. Enzymatic Catalysis: Enzymes, the largest class of proteins, catalyze biochemical reactions. Examples include hexokinase, which initiates glucose metabolism, and DNA polymerase, which facilitates DNA replication.
3. Storage Function: Some proteins store amino acids for growth and development. Examples include ovalbumin (egg white protein), casein (milk protein), and gliadin (wheat protein).
4. Transport Function: Proteins transport molecules throughout the body. Examples include:
i. Serum albumin, which binds and transports fatty acids.
ii. Lipoproteins, which transport lipids between tissues.
iii. Hemoglobin, which carries oxygen in vertebrates.
iv. Haemocyanin, an oxygen carrier in invertebrates.
5. Contractile and Motile Function: Actin and myosin are major proteins in muscle contraction, sliding past each other to enable movement.
6. Protective Role: Proteins contribute to immune defense. Examples include:
i. Thrombin and fibrinogen, which aid blood clotting.
ii.Antibodies (immunoglobulins), which neutralize harmful foreign substances.
7. Toxic Proteins: Some plant proteins act as toxins, such as ricin (castor beans), gossypol (cotton seeds), and haemagglutinins (legumes). Certain protein-based viruses also cause diseases in plants and animals.
8. Hormonal Regulation: Some proteins act as hormones, regulating physiological processes. Examples include:
i. Growth hormone (Somatotropin), secreted by the pituitary gland.
ii. Insulin, produced by the pancreas to regulate glucose metabolism. Its deficiency leads to diabetes mellitus.
9. Unique Functions: Some proteins perform specialized roles:
i. Fibroin, produced by spiders and silkworms, forms strong silk threads.
ii. Antifreeze proteins, found in Antarctic fish, prevent blood from freezing.
iii. Monellin, a sweet-tasting protein found in fruits, provides sweetness without contributing to fattening.
Proteins possess four fundamental structural levels (primary, secondary, tertiary, and quaternary) and display distinctive properties such as amphoterism, colloidal behavior, and denaturation. Their classification into simple, conjugated, and derived proteins helps in understanding their function and significance in agriculture and biological sciences.
Do you have any questions, suggestions, or contributions? If so, please feel free to use the comment box below to share your thoughts. We also encourage you to kindly share this information with others who might benefit from it. Since we can’t reach everyone at once, we truly appreciate your help in spreading the word. Thank you so much for your support and for sharing!

